FUELL CELLS
These investigations concern cell dynamics (in this case fuel cells), we also study how water acts a barrier for oxygen transport in fibers. The result of the liquid water after the stacked fiber screens have been exposed to it are that it acts as a barrier for motion, and results in decreasing available cross sectional area. Below can be seen the effect on the stacked fiber screens of a cell after it is exposed to a water-vapor saturated atmosphere.
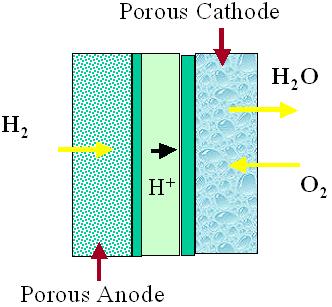
As well, ion conductivity also takes place in the membrane, and some of the processes that take place include production and transportation.
Production of:
– Micro-droplets, and macro-droplets
– Formation of surface droplet
Transportation of:
– In the diffusion layer, the Capillary mechanism
– In the channel, the Drag force
There are two types of droplets: Pinned Droplets and Sliding droplets.
The pinned portion of the droplet does not allow the portion close to the contact line to deform.
The sliding droplet reduces the relative velocity between the ambient fluid, resulting in lower deformation
FIRE SPREAD
Combustion and fire spread is one of the important safety issues in any location where there are combustible material. We have been conducting experimental research on the combustion and flame spread of variety of fuel, including, emulsified fuels.
Emulsions are a two phase system, consisting of droplets (internal phase) dispersed throughout a continuous (external) phase. For the case of an oil-in-water (O/W) emulsion, the dispersed phase is oil and the continuous phase is water. The structure is thermodynamically unstable, so that a surfactant (surface-active agent) must be added to stabilize the system. Emulsions exhibit a high yield stress and a much higher apparent viscosity than either of their two components. For these reasons, they are not prone to slosh, splash, or spill. (The following figures show a sequence of flame spread as conducted in an experiment).
 |
 |
 |
These characteristics of emulsions led to study of their application as fuels in military aircraft. Results of tests in gas turbine engines showed that the pumpabilitiy of emulsified fuels was only slightly lower than that of the liquid fuel. In addition, there was no significant reduction in engine performance with these fuels. The only problem was the corrosion and rust of parts susceptible to such effects through prolonged exposure to water. More recently, as explained above, emulsified fuels have been seen as a solution to the problem of safe transport of liquid hazardous wastes. Research has shown that the emulsions have a flame suppression characteristic at certain temperatures, and at certain concentrations of the inner fuel phase. At these critical points, the flame propagation rate across a fuel reduces up to two orders of magnitude. Thus, the safety of such a substance in the case of an accidental spill is easily seen. In addition, research with W/O emulsions has shown an improved efficiency in the combustion of these substances, due to the onset of “microexplosions”. Along with ease of cleanup due to their high viscosity, these characteristics make emulsified fuels an attractive option for the safe transport of liquid hazardous waste. We have been conducting flame spread experiments on O/W emulsions to determine the flame suppression characteristics of emulsions at certain temperatures and concentrations. We have developed models for the mechanisms of flame propagation across an emulsified fuel.
INCINERATION OF HAZARDOUS LIQUID WASTES
Incineration of hazarous liquid waste invesitgated through combustion studies of three chlorobenzenes (monochlorobenzene, dichlorobenzene, trichlorobenzene) and five alkanes (octane, decane, dodecane, tetradecane, and hexadecane) is being investigated. Using high-speed video photography, the size and velocity of the burning drops at various points during their lifetime were measured. From such data, the time-variation of a droplet’s burning rate was deduced. We are finding out that the variations of the burning rates for the mixtures were qualitatively similar when viewed as functions of the chlorine to hydrogen atom ratio. Starting from a pure alkane, as Cl/H increased, the burning rate first decreased, then slightly increased, and then fell sharply near Cl/H = 0.5 to approximately the vaporization rate of the pure chlorobenzene. We can also see that prior droplet combustion studies (performed in the water-rich atmosphere of combustion-heated reactors) exhibited burning rates of the same liquid blends that were nearly twice the present dry atmosphere results. We have demonstrated that when the hydrogen in the surrounding atmospheric water is included in the ratio Cl/H, these previous results yield curves of burning rate vs. Cl/H that are similar to those of the present study. This implies that the enhanced burning rate observed when an alkane is blended into a pure chlorobenzene is partly due to the hydrogen provided by the alkane, and that a similar effect appears achievable by adding water vapor to the gas phase.
COMBUSTION OF EMULSIONS
Emulsions are a two phase system, consisting of droplets (internal phase) dispersed throughout a continuous (external) phase. For the case of an oil-in-water (O/W) emulsion, the dispersed phase is oil and the continuous phase is water. The structure is thermodynamically unstable, so that a surfactant (surface-active agent) must be added to stabilize the system. Emulsions exhibit a high yield stress and a much higher apparent viscosity than either of their two components. For these reasons, they are not prone to slosh, splash, or spill. These characteristics of emulsions led to study of their application as fuels in military aircraft. Results of tests in gas turbine engines showed that the pumpabilitiy of emulsified fuels was only slightly lower than that of the liquid fuel. In addition, there was no significant reduction in engine performance with these fuels. The only problem was the corrosion and rust of parts susceptible to such effects through prolonged exposure to water.
More recently, as explained above, emulsified fuels have been seen as a solution to the problem of safe transport of liquid hazardous wastes. Research has shown that the emulsions have a flame suppression characteristic at certain temperatures, and at certain concentrations of the inner fuel phase. At these critical points, the flame propagation rate across a fuel reduces up to two orders of magnitude. Thus, the safety of such a substance in the case of an accidental spill is easily seen. In addition, research with W/O emulsions has shown an improved efficiency in the combustion of these substances, due to the onset of “microexplosions”. Along with ease of cleanup due to their high viscosity, these characteristics make emulsified fuels an attractive option for the safe transport of liquid hazardous waste.
MICRO-EXPLOSION
The explosion dynamics of a liquid drop driven by a high-pressure bubble is simulated numerically by solving the full two-dimensional Navier-Stokes equations and exact boundary equations. It is shown that bubble surface roughness generated by Landau instability during the rapid evaporation stage of the internal phase significantly affects the hydrodynamics instability of the subsequent stage. This study suggests that Rayleigh-Taylor instability controls the hydrodynamics of the subsequent stage and affects the microexplosion disruptive phenomenon and the drop breakup time. The numerical investigation demonstrates the effects of surface tension, viscosity, pressure and size of the internal phase, and characteristics of interfacial disturbance on the internal phase growth, the bubble surface phenomena and the drop breakup time.
The phenomenon of microexplosion is often observed during the combustion of fuel emulsions and multi-component fuel droplets, which are made up of two or more liquids with relatively large differences between their boiling temperatures. The microexplosion may occur when the superheat limit of the interior phase (which is far above the boiling point and is about 10% below the critical temperature for many substances) is lower than the saturation temperature of the surrounding phase. Intense disruption occurs due to the rapid evaporation of the droplet interior phase by spontantaneous homogeneous nucleation which results in a high pressure bubble inside the liquid phase (Shepherd and Sturtevant 1982). The bubble grows violently causing the disruption of the liquid drop into small secondary drops in a process known as secondary atomization or microexplosion. The concentration and physical properties of the droplet components as well as the size of the internal drops and their interior locations determine the severity of the liquid disruption.
The microexplosion of fuel droplets is very effective in reducing the unburned soot particles, which are formed when burning heavy fuel oils, by producing smaller fuel droplets and better spray dispersion. It has been observed that the bubbles generated by homogeneous nucleation have large amplitude small-scale rough surface during most of the evaporative stage which is in contrast of the smooth bubbles observed in conventional boiling and is attributed to the Landau instability accompanying the rapid evaporation process. After the rapid evaporation of the internal phase, a liquid droplet entrapping a high-pressure bubble with small-scale perturbation at their interface is expected.
